Group in the periodic table. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table.
What Is The Definition Of Halogen In Chemistry, In the production of polymers, drugs. Halogen definition, any of the electronegative elements, fluorine, chlorine, iodine, bromine, and astatine, that form binary salts by direct union with metals.

The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i), astatine (at), and tennessine (ts). Definition of ~ s 1) group viia elements: Halogen definition, any of the electronegative elements, fluorine, chlorine, iodine, bromine, and astatine, that form binary salts by direct union with metals. These elements are called the halogens (from the greek hals, salt, and gennan, to form or generate) because they are literally the salt formers.
These elements are called the halogens (from the greek hals, salt, and gennan, to form or generate) because they are literally the salt formers.
There are five halogens in the periodic table of chemical elements: Halogens are found in the environment only in the form of ions or compounds, because of. Fluorine (f), chlorine (cl), bromine (br), iodine (i), and astatine (at). Group in the periodic table. Redox reactions as the halogen is an oxidising agent but not itself reduced. Halogens the halogens are a group of elements in the periodic table.
 Source: thechemistryguru.com
Source: thechemistryguru.com
They also form anions like the hypochlorite, chlorate, perchlorate, and bromate ions that are very strong oxidizing agents. A halogen is a chemical element that forms a salt when it reacts with metal. For more information, see the related links, below. In the modern iupac nomenclature, this group is known as group 17. Xenon is a noble gas that has.
 Source: thoughtco.com
Source: thoughtco.com
Consider the hypothetical reaction $\mathrm { a } ( g ) + 2 \mathrm { b } ( g ) \rightleftharpoons 2 c(g),$ for which $k_c = 0.25$ at a certain temperature. Halogens are found in the environment only in the form of ions or compounds, because of. Definition of halogen (entry 1 of 2) : These elements are called.
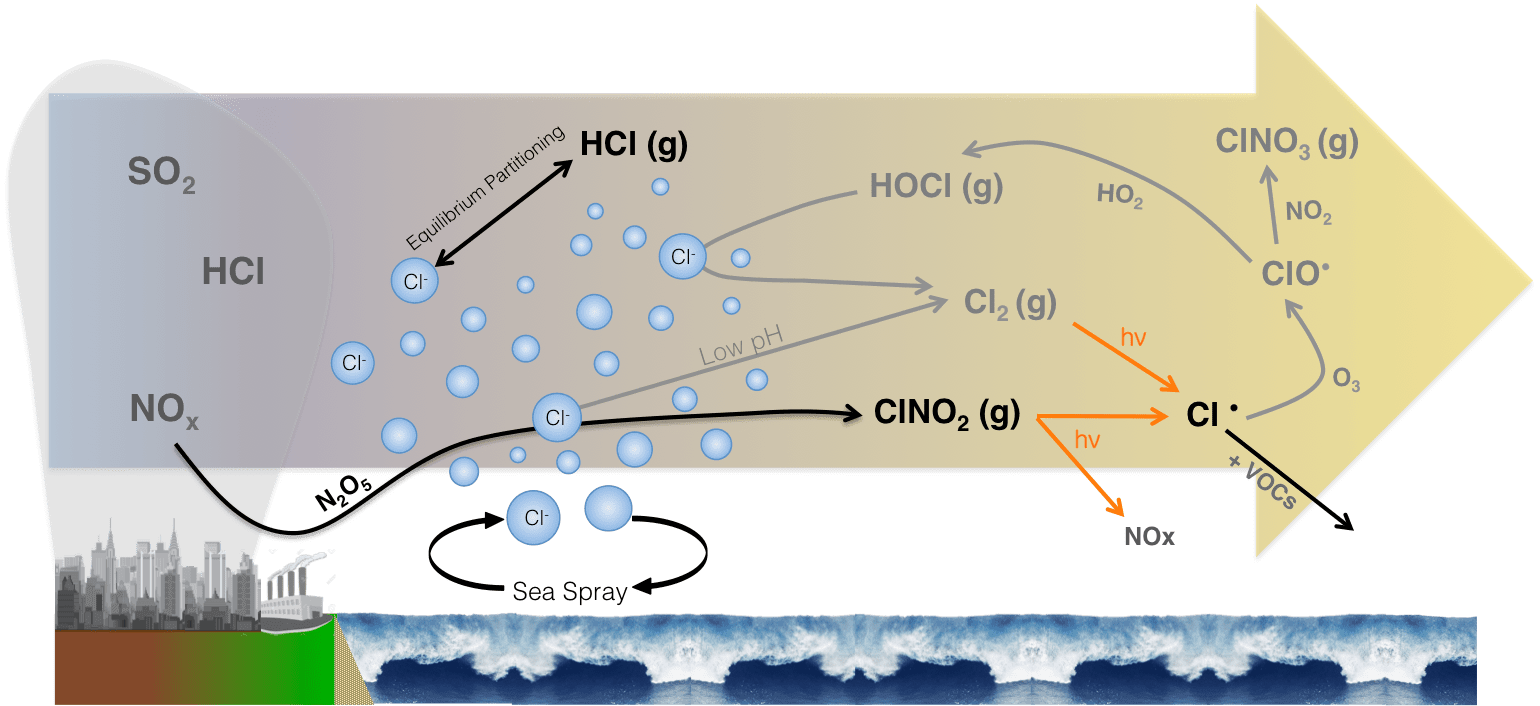 Source: atmos.washington.edu
Source: atmos.washington.edu
Elements in the halogen group have seven electrons in their outer shells giving them many unique properties. Thinking in this way suggests it is a halogen bond. In the modern iupac nomenclature, this group is known as group 17. Definition of ~ s 1) group viia elements: The halogen elements are fluorine, chlorine, bromine, iodine, astatine, and possibly tennessine.
 Source: thoughtco.com
Source: thoughtco.com
They form oxoacids in four different types with general formula (hox). Halogen refers to the chemical elements in the group 7 of the periodic table of elements. Halogens are highly chemically reactive. The halogens are nonmetallic and not reactive. The halogens are the elements in group 17 of the periodic table.
 Source: chemizi.blogspot.com
Source: chemizi.blogspot.com
They form oxoacids in four different types with general formula (hox). The halogens are nonmetallic and not reactive. Terms in this set (19) the halogens. Definition of halogen (entry 1 of 2) : The halogen elements are fluorine, chlorine, bromine, iodine, astatine, and possibly tennessine.
 Source: youtube.com
Source: youtube.com
Variable noun [oft n n] a halogen is one of a group of chemical elements that includes chlorine, fluorine, and iodine. Fluorine, chlorine, bromine, iodine, and astatine. Halogens the halogens are a group of elements in the periodic table. A halogen is a chemical element that forms a salt when it reacts with metal. The halogens are the elements in.
 Source: utedzz.blogspot.com
Source: utedzz.blogspot.com
The halogens are nonmetallic and not reactive. Xenon is in the group 8 of the periodic table. Halogen as a noun means any of a group of five chemically related nonmetallic elements including fluorine, chlorine, bromine, iodine, and astati. The halogens are all highly reactive, which means they�re quick to form bonds withother. Fluorine occurs in fluorite, caf2, and other.
 Source: sciencenotes.org
Source: sciencenotes.org
Definition estimation of amount of halogen a known mass of an organic compound is heated with fuming nitric acid in the presence of silver nitrate contained in a hard glass tube, known as carius tube, in a furnace.carbon and hydrogen present in the. A halogen is a gas, such as iodine or bromine. The artificially created element 117, tennessine (ts),.

The elements are found in nature with salt, nacl, being the most abundant compound of chlorine. The halogens are a group of elements in the periodic table. Halogen bulbs contain a small amount of halogen gas. The halogens are the elements in group 17 of the periodic table. They are located to the right of the other nonmetals and to.
 Source: youtube.com
Source: youtube.com
Halogens the halogens are a group of elements in the periodic table. The halogens are all highly reactive, which means they�re quick to form bonds withother. A halogen is a chemical element that forms a salt when it reacts with metal. Halogen bulbs contain a small amount of halogen gas. They are located to the right of the other nonmetals.
 Source: slideshare.net
Source: slideshare.net
Terms in this set (19) the halogens. Elements in the halogen group have seven electrons in their outer shells giving them many unique properties. Halogen refers to the chemical elements in the group 7 of the periodic table of elements. The halogens are reactive elements that consist of diatomic molecules. This kind of conversion is in fact so common that.
 Source: chem.ucla.edu
Source: chem.ucla.edu
The halogen elements are fluorine (f), chlorine (cl), bromine (br), iodine (i), astatine (at), and tennessine (ts). Fluorine occurs in fluorite, caf2, and other minerals. The halogens are all highly reactive, which means they�re quick to form bonds withother. Halogens are in the group 7 of the periodic table. Definition estimation of amount of halogen a known mass of an.
 Source: konongo-mampong-diocese.org
Source: konongo-mampong-diocese.org
Elements in the halogen group have seven electrons in their outer shells giving them many unique properties. Redox reactions as the halogen is an oxidising agent but not itself reduced. Halogens are found in the environment only in the form of ions or compounds, because of. There are five halogens in the periodic table of chemical elements: They also form.
 Source: pinterest.com
Source: pinterest.com
Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Consider the hypothetical reaction $\mathrm { a } ( g ) + 2 \mathrm { b } ( g ) \rightleftharpoons 2 c(g),$ for which $k_c = 0.25$ at a certain temperature. Halogen chemistry the halogens react violently with alkali metals, alkaline earth.
 Source: periodictableguide.com
Source: periodictableguide.com
Fluorine, chlorine, bromine, iodine, and astatine. None of the halogens can be found in nature in their elemental form. The nucleus of the halogen (chlorine for example) is much stronger at withdrawing electrons than the hydrogen nucleus. The halogens are highly reactive nonmetallic elements. Halogens are found in the environment only in the form of ions or compounds, because of.
 Source: byjus.com
Source: byjus.com
Consider the hypothetical reaction $\mathrm { a } ( g ) + 2 \mathrm { b } ( g ) \rightleftharpoons 2 c(g),$ for which $k_c = 0.25$ at a certain temperature. This kind of conversion is in fact so common that a comprehensive overview is challenging. Thinking in this way suggests it is a halogen bond. Halogens are highly.
 Source: byjus.com
Source: byjus.com
Halogen refers to the chemical elements in the group 7 of the periodic table of elements. Xenon is a noble gas that has the symbol xe. Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Xenon is in the group 8 of the periodic table. Halogens are highly chemically reactive.
![Chemistry Halogen [PPTX Powerpoint] Chemistry Halogen [PPTX Powerpoint]](https://i2.wp.com/static.fdocuments.in/img/1200x630/reader020/image/20191006/546da58ab4af9fe51b8b4f2e.png?t=1601758656) Source: fdocuments.in
Source: fdocuments.in
The halogens are highly reactive nonmetallic elements. Fluorine (f), chlorine (cl), bromine (br), iodine (i), and astatine (at). Xenon is in the group 8 of the periodic table. The halogens are all highly reactive, which means they�re quick to form bonds withother. The halogens are a group of elements in the periodic table.

Elements in the halogen group have seven electrons in their outer shells giving them many unique properties. Terms in this set (19) the halogens. The halogens are a group of elements in the periodic table. Oxoacids of halogen are compounds that contain at least one oxygen, hydrogen and no less than one other halogen (group 17 elements include fluorine, chlorine,.

Fluorine occurs in fluorite, caf2, and other minerals. For more information, see the related links, below. Redox reactions as the halogen is an oxidising agent but not itself reduced. Halogens the halogens are a group of elements in the periodic table. None of the halogens can be found in nature in their elemental form.
 Source: physciq.blogspot.com
Source: physciq.blogspot.com
Halogens are often used in lighting and heating devices. Fluorine, chlorine, bromine, iodine, and astatine. There are five halogens in the periodic table of chemical elements: Fluorine (f), chlorine (cl), bromine (br), iodine (i), and astatine (at). The artificially created element 117, tennessine (ts), may also be a halogen.
 Source: britannica.com
Source: britannica.com
Halogens are highly chemically reactive. Oxoacids of halogen are compounds that contain at least one oxygen, hydrogen and no less than one other halogen (group 17 elements include fluorine, chlorine, bromine, iodine, and astatine which are known as halogens). There are five halogens in the periodic table of chemical elements: Fluorine, chlorine, bromine, iodine, and astatine. Xenon is a noble.
 Source: showme.com
Source: showme.com
The halogens are a group of elements in the periodic table. There are five halogens in the periodic table of chemical elements: Fluorine (f), chlorine (cl), bromine (br), iodine (i), and astatine (at). There are five halogens in the periodic table of chemical elements: This kind of conversion is in fact so common that a comprehensive overview is challenging.
 Source: aim2destiny.blogspot.com
Source: aim2destiny.blogspot.com
For more information, see the related links, below. There are five halogens in the periodic table of chemical elements: There are five halogens in the periodic table of chemical elements: Halogen as a noun means any of a group of five chemically related nonmetallic elements including fluorine, chlorine, bromine, iodine, and astati. The halogens are all highly reactive, which means.
 Source: youtube.com
Source: youtube.com
Halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. The halogens are highly reactive nonmetallic elements. In the production of polymers, drugs. Halogen bulbs contain a small amount of halogen gas. A halogen is a chemical element that forms a salt when it reacts with metal.